 Prior to MEDDEV 2.7/1 rev 4, guidance referred to state of the art only vaguely. MEDDEV 2.7/1 rev 3 requested the discussion of clinical data “in comparison with” and “taking account of” state of the art, and that the clinical literature data cited “reflect current medical practice and the generally acknowledged state of the art technologies”. From a methodological standpoint, this is not much guidance. Thus, methodology, depth, and presentation of the medical background for a device was largely left to one’s interpretation, and thus conducted inconsistently, or, at least, heterogeneously.
Prior to MEDDEV 2.7/1 rev 4, guidance referred to state of the art only vaguely. MEDDEV 2.7/1 rev 3 requested the discussion of clinical data “in comparison with” and “taking account of” state of the art, and that the clinical literature data cited “reflect current medical practice and the generally acknowledged state of the art technologies”. From a methodological standpoint, this is not much guidance. Thus, methodology, depth, and presentation of the medical background for a device was largely left to one’s interpretation, and thus conducted inconsistently, or, at least, heterogeneously.
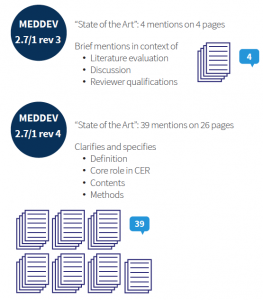
MEDDEV 2.7/1 rev 4 not only clarifies the definition and purpose of establishing state of the art, but also includes guidance on methodology and required content. The revision expands substantially on the four very brief mentions of “state of the art” in the previous guidance (see the figure below). A total of 39 mentions on 26 pages of the MEDDEV 2.7/1 rev 4 document not only provides a comprehensive understanding of the meaning and significance of state of the art, but also details requirements of how to establish and document this information.
According to MEDDEV 2.7/1 rev 4, state of the art describes what is currently and generally considered standard of care, or best practice, for the medical condition or treatment for which the device is used. Analysis and description of the state of the art provides the context in which the manufacturer (and Notified Body) can assess the safety and performance of the device, and determine the acceptability of its benefits and risks, in comparison to other available therapeutic options. In the presentation and whitepaper, we look into the importance of state of the art, and present practical approaches to developing the state of the art in more detail.
If you want to find out more about State of the Art according in MEDDEV 2.7/1 rev 4, you can download our full whitepaper.

 Prior to MEDDEV 2.7/1 rev 4, guidance referred to state of the art only vaguely. MEDDEV 2.7/1 rev 3 requested the discussion of clinical data “in comparison with” and “taking account of” state of the art, and that the clinical literature data cited “reflect current medical practice and the generally acknowledged state of the art technologies”. From a methodological standpoint, this is not much guidance. Thus, methodology, depth, and presentation of the medical background for a device was largely left to one’s interpretation, and thus conducted inconsistently, or, at least, heterogeneously.
Prior to MEDDEV 2.7/1 rev 4, guidance referred to state of the art only vaguely. MEDDEV 2.7/1 rev 3 requested the discussion of clinical data “in comparison with” and “taking account of” state of the art, and that the clinical literature data cited “reflect current medical practice and the generally acknowledged state of the art technologies”. From a methodological standpoint, this is not much guidance. Thus, methodology, depth, and presentation of the medical background for a device was largely left to one’s interpretation, and thus conducted inconsistently, or, at least, heterogeneously.