The large number of mentions of state of the art throughout MEDDEV 2.7/1 rev 4 provide not only a comprehensive description of the importance, purpose, and role of establishing the state of the art, but also inform on how to incorporate this analysis into the clinical evaluation. The image below summarizes several core roles of this analysis.
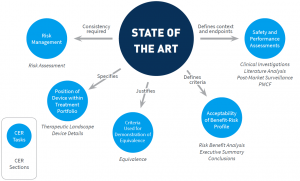
Establishing and describing state of the art is not an isolated task, but is central to the entire clinical evaluation. Defining the current, accepted best treatment options, and describing the risks and benefits of these options, provides essential information for multiple aspects of the clinical evaluation. Thus, establishing state of the art yields information that is essential for determining if the safety and performance of a device is compatible with current standards (in comparison to available treatment options). In other words, state of the art establishes a reference standard that is used throughout the clinical evaluation.
If you want to find out more about State of the Art according in MEDDEV 2.7/1 rev 4, you can download our full whitepaper.


