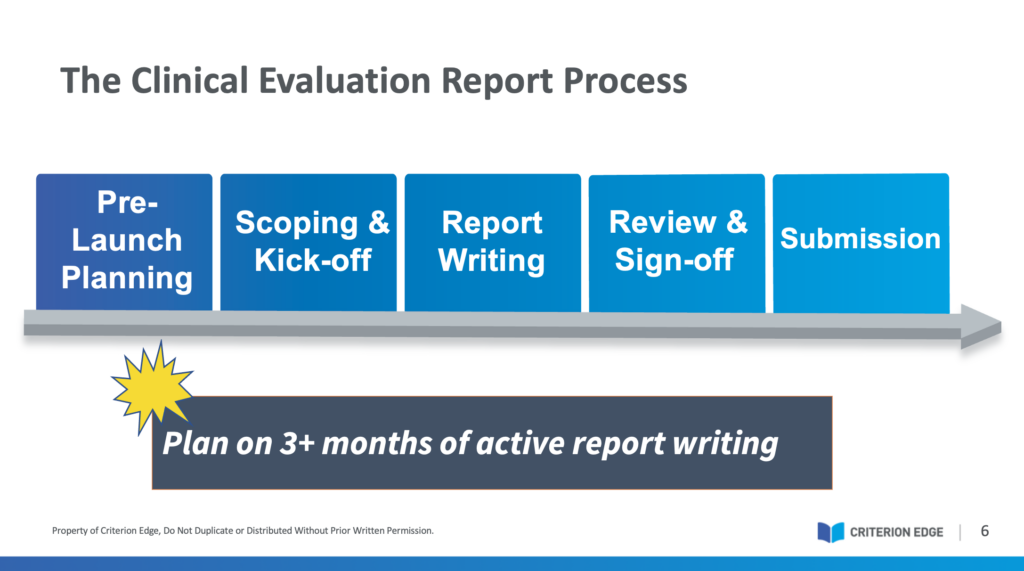
The goal of performance evaluation is to show that your IVD performs as intended and meets the acceptance criteria, therefore establishing that your IVD may be considered state of the art. Remember: the PER is a product, but it is documenting a process. Performance evaluation can be straightforward if your analyte has a well-established association with a clinical condition. But what is the purpose of the SVR, and how should you approach it when there is too much or not enough data?
This webinar will discuss the role of data sufficiency, the importance of developing a solid strategy to establish scientific validity, how gaps in the SVR can significantly impact the quality of the PER, and how proper scoping can help maximize success when it comes to a performance evaluation.
In this webinar, you will learn
1. How to clearly define what information should (and shouldn’t) be presented within the Scientific Validity Report.
2. How the systematic literature review process supports and connects the SVR, CPR, and APR by leveraging published data.
3. How to conduct gap assessments to assess IVDR readiness and develop strategies to increase efficiencies in the performance evaluation process to support the SVR.
At the end of the presentation, we will dedicate 15 minutes to answering your questions. Please submit your questions beforehand if you have any to help us prepare. Also, you can submit your questions at any point during the presentation.
Who should register for this webinar?
Click here to watch the recording of this webinar.
New Requirements for In Vitro Diagnostics: Lessons Learned from the AAMI/FDA/BSI Annual Conference
In October 2022, the AAMI/FDA/BSI Annual Conference discussed current knowledge and practical strategies to meet new IVDR clinical evidence requirements. Dr. Sarah Chavez, Criterion Edge Director of IVD Writing Services, was invited to speak with other regulatory, notified body and industry leaders as panelists at the pre-conference workshop “New Requirements for In Vitro Diagnostics”.
Watch Dr. Chavez’s recent webinar and benefit from key insider insights gleaned from this meeting. Sarah shares and discusses the key take-aways from the Symposium, including clinical evidence requirements and strategies to keep your report writing and data collection on track. Many companies are in the early stages of planning for IVDR, so take a step towards IVDR readiness with these helpful suggestions for planning and preparation from the perspective of experienced regulatory writers.
Watch the webinar recording here.