Author: Nupur Srivastava, PhD | November 18, 2020
There has been a lot of discussion on the updated International Organization for Standardization (ISO) 14971:2019, and experts agree that medical device manufacturers need to perform a gap analysis and create a cross-functional team to meet these risk management requirements.
Risk management plays a critical role in getting medical devices into the market, and ISO 14971:2019 “was designed to better align with medical device regulations around the world.” The global regulatory landscape has been very dynamic around the areas of risk management, as well as safety and performance.
Tips on Putting Risk Management into Quality Systems During the Transition to ISO 14971:2019
Two experts shared insights during an interview with Medtech Insight. Jos Van Vroonhoven, convener of ISO Technical Committee 210, Joint Working Group 1 (TC210/JWG1) and a senior manager for standardization for device giant Philips Healthcare, and Don Powers, a member of ISO TC210/JWG1 and long-time industry expert, said that the third edition of ISO 14971 provides more precise guidance and greater detail in the application of risk management concepts.
These experts provided specific tips on how to transition ISO 14971:2019 into your device’s quality systems.
Since ISO 14971:2019 instructs manufacturers how to best put together risk management programs, this is a good time to review risk management procedures and perform Gap Assessments.
- Develop a Gap Assessment Team
- Led by quality assurance or a designated risk manager
- Consisting of members with expertise in:
- Clinical knowledge of the device
- Regulatory knowledge, global if needed
- Manufacturing of the device
- Design and development
- Marketing
- Reliability engineer and service organization, if available
- Divide the Gap Assessment Team and Conquer Each Area
- Compare required ISO 14971:2019 to current company processes
- Determine how this will impact each department
- Document Risk Management to Evaluate Residual Risk
- The panelist placed particular emphasis on residual risk, or the risk that remains after risk control measures have been implemented, which is new in ISO 14971:2019. Evaluating and Setting criteria for risk management can be found in Clause 4.4e Risk Management.
How Do You Know If You are On the Right Track?
Create another team to evaluate residual risks. This group needs to form a cross-functional team of experts with application and clinical knowledge that can provide good judgment for the residual risk of the medical device.
The example provided explored the residual risk of x-ray equipment. In this case, the residual risk team might be comprised of individuals with expertise in the following areas.
- Radiation
- Technical director
- Chief medical officer
- Clinical scientist that understands how the equipment is used in hospitals.
The standards outlined in ISO 14971:2019, like benefit-risk analysis, are now in demand by global regulators.
Global Regulations
The ISO 14971 standards were updated in 2019 in the United States and the European Union to conform to the higher expectation of identifying and mitigating risks associated with a medical device throughout its lifecycle.
In the US, FDA requires device manufacturers to align with ISO 14971:2019 by the end of the transition period December 25, 2022, after which time FDA will no longer accept the previous ISO 14971:2007 version.
In Europe, ISO 14971:2019 (EN version) aligns with the Medical Device Regulations (MDR) and In Vitro Diagnostic Regulation (IVDR) safety and performance requirements. Although it is not yet harmonized with these standards, ISO 14971:2019 is expected to become a Harmonized standard, which will make it state-of-the-art. The date of application for MDR (26 May 2021) and IVDR (26 May 2022) is quickly approaching.
Globally, ISO 14971:2019 is considered the international standard for risk management and is referenced, if not endorsed, by all other major markets that require the application of risk management including, Australia TGA, Brazil, Health Canada, and Japan MHLW.
Therefore, device manufacturers must begin to transition into compliance with the revised version of ISO 14971:2019 to align with global regulations.
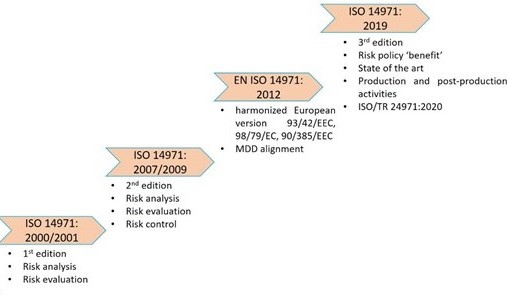
History of ISO 14971
The first edition of ISO 14971 was released in 2000 to assist manufacturers, regulatory bodies, and healthcare providers in managing the medical devices’ potential risks. This picture depicts the evolution of ISO 14971 from 2000 to 2019.
As global regulations for medical devices are becoming more stringent, clear documentation of the process and presentation of the data are essential components of getting medical devices to market. Criterion Edge has a team of expert medical writers that can help your organization get the required documents completed in a timely manner.